Trends in Study Design
In bringing novel therapeutics to market, clinical development programs face intense pressures to meet speed and efficiency goals. Enter innovative adaptive study designs that enable modification of the clinical program, drawing from early efficacy readouts. Such approaches aim to avoid sunken costs and reallocate resources to the most viable candidate therapeutics or research questions of interest with the aim of accelerating the availability of efficacious treatments to patients. As a consequence, clinical study designs are gaining complexity to answer as many questions within a single study.
Many factors are driving this complexity – such as rapid technological and scientific advancements in various fields, greater interest in rare disease research, shifts to decentralized or hybrid study designs, proliferation of data sources (e.g.., biological samples, ePRO, trackers) and the incorporation of real-world evidence for natural history or synthetic control arm data. Together, these influences contributed to prolonging the average clinical study follow-up by ~6.7 months from 2010 to 2020.1 Additionally, adaptive trials by design collect far more study data compared to traditional studies, requiring additional expertise and labor resources to analyze data and prepare study reports. Below we highlight some of the key considerations and industry trends pertaining to research study design and execution.
Consider the complexity of the study design
The largest driving factor in the complexity of study designs has been the shift towards master or adaptive protocol study designs that can answer several questions at once to steer the program forward. Many drug development programs have shifted away from traditional phase 1/2/3 study designs to reduce study start-up cost and time, while also trying to answer as many research questions within a single study. Most commonly, this is achieved with master protocol designs that can evaluate one or more investigational product in one or more disease subtypes, with additional sub-studies incorporated into the overall design. The master protocol can be designed as a fixed or adaptive protocol, where interim data analyses can help steer the design towards the addition or termination of other research arms or questions to be answered.
Overall, with the added complexity of a master protocol or adaptive study design, each successive study can be better informed to optimize various parameters, including outcome measures, patient subpopulation recruitment and sample or data collection timepoints. The greatest study design complexity is being seen in oncology studies, which tend to have the greatest number of data points and study arms, typically to accommodate testing various combination therapeutics in certain cancer type subpopulations. To support adaptive and master protocol study designs, Sanguine has developed a flexible condition access model, which can streamline complex clinical program needs and further accelerate clinical program timelines.
Access to target population
As research studies expand into a wide variety of disease fields, including gene therapies to treat rare diseases, contacting and recruiting eligible research participants becomes paramount. Such sentiment was reflected in a recent survey we conducted with 100 translational and clinical researchers. When asked which study criteria were most important, access to a wide group of patients was the most cited response. (Figure 1).
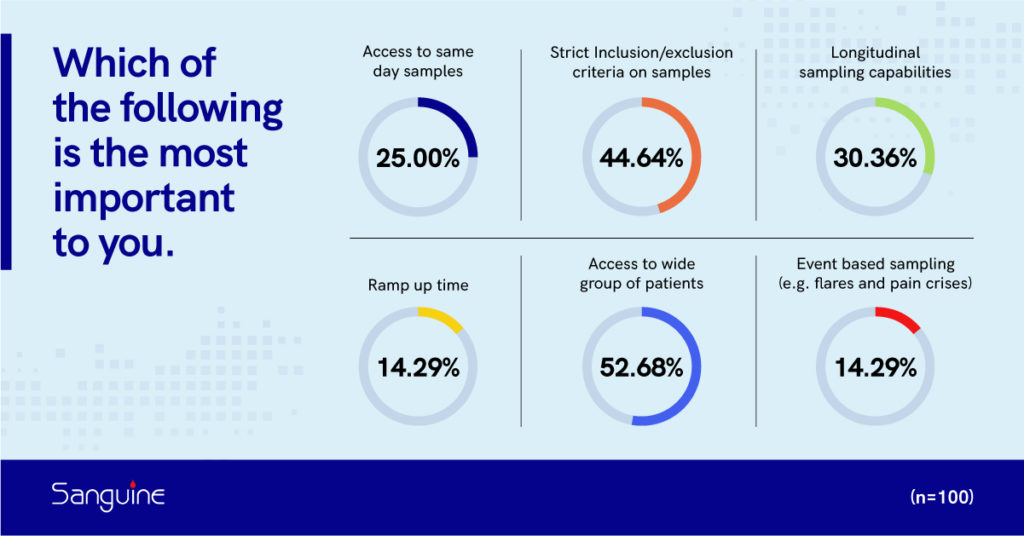
Figure 1. Researcher responses to the question of which three study criteria are most important for your study needs. (n=100)
Sanguine’s patient-centered approach uniquely connects researchers with an enthusiastic patient population. Among our growing database of >50,000 engaged potential participants across the United States are patients with rare diseases who are often difficult to recruit. By building mutually beneficial relationships with patient advocacy groups (PAGs) and treating patients like partners in the research process, we continuously welcome new study participants who are committed to completing studies and stay involved with future opportunities. Building bonds with PAGs fosters an environment focused on education, informed consent and incorporating patient perspectives into research.
Optimize Eligibility Criteria
Technological advances (e.g., ‘omics’ and imaging technologies) are facilitating the discovery of novel biomarkers that inform on the likelihood of patient response to therapeutics. As a result, studies often stratify participants into specific subpopulations based on predictive response to more targeted therapies, which is driving stricter inclusion and exclusion participant criteria to ensure study success. As such, survey respondents also cited setting strict inclusion and exclusion criteria to recruit the ideal study participants as a key component of study design (Figure 1). Recent trends are demonstrating greater and greater selectivity to stratify participants into specific subpopulations. Studies focusing on polygenic diseases or diseases with a high degree of phenotypic diseases heterogeneity, such as autoimmune diseases, are seeing great success in stratification based on molecular biomarkers. In this way, novel therapeutics that only work for a certain subpopulation can be developed, allowing for t more personalized approaches to medical care that are improving patient outcomes and quality of life.
Two popular study designs, the umbrella and basket study approaches, are often employed when patient stratification is desired. In an umbrella design, participants with a certain biomarker or molecular signature related to a single disease are stratified to receive multiple interventions. By contrast, the goal of a basket study design is to identify among patients with diverse disease (e.g., cancers of different tissues) a subpopulation who share a common biomarker or genetic mutation that is indicative of response to a targeted treatment. Such basket designs can also be applied to rare disease research where the same mutations manifest as different phenotypes, and therefore different syndromes in patients. By categorizing these patients based on their molecular phenotypes and not their specific disease name, a greater number of patients can be enrolled and potentially treated with the same therapeutic approach.
Based on the desired eligibility criteria, Sanguine can provide feedback on enrollment feasibility and screen failure estimates. Our patient network is comprehensively annotated with relevant patient medical history and other data to allow us to target enrollment and recruit participants rapidly and effectively, with limited geographical restraints. Medical records often include confirmed patient diagnoses, past and current treatments, diagnostic lab or assay results, and medical scans or testing imagery. This level of annotation enables efficient recruitment of the right participants to your study in a timely manner. In-home collection also permits rapid screening with predetermined molecular tests, further facilitating enrollment of eligible and willing study participants.
Determine sample and data collection needs
Researchers we surveyed identified that some of the biggest challenges in enrolling patients into a study included: the motivation to participate, retaining patients, the frequency of visits and the distance to travel for the visit (Figure 2).
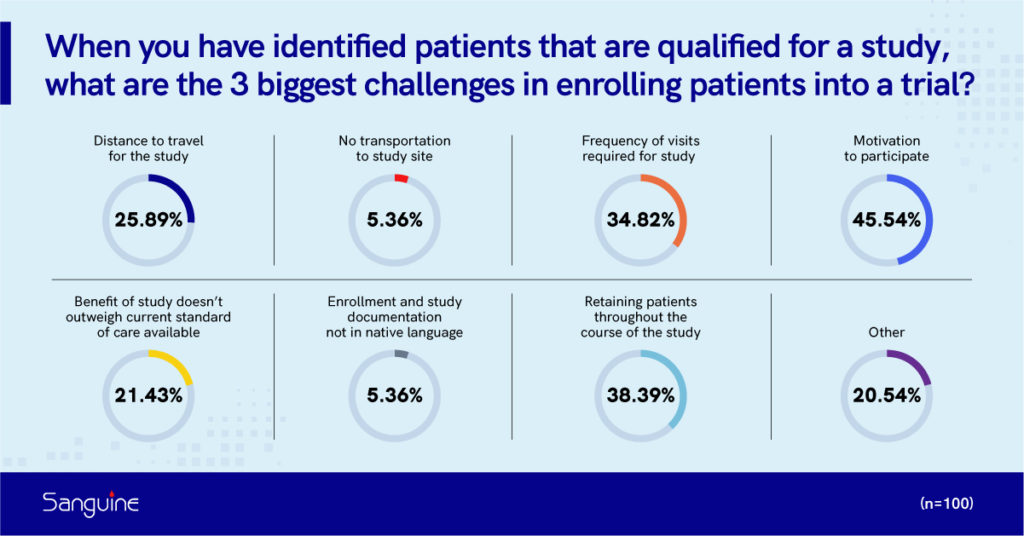
Figure 2. Researcher respondents identify the biggest challenges associated with enrolling patients into a study. (n=100)
Sanguine’s mobile at-home approach substantially reduces the burdens of study participation, and builds relationships with our patients to keep them informed about emerging research, motivating them to participate in studies they may be eligible for. This enables researchers to rapidly enroll eligible participants who are comfortable at the time of collection into carefully designed studies. Our commitment to our patient populations is one of the leading reasons that we are able to rapidly enroll patients into our studies and maintain an exceptionally high study retention rate of >90% across most of our studies.
As the complexity and sensitivity of downstream analyses increases, researchers are often requesting same-day delivery and limited sample manipulation to reduce the interference of artifacts in the sample data. More studies are now also incorporating patient reported outcomes (PRO) with the ease of electronic devices to administer custom or validated questionnaires. Such data can provide valuable patient perspectives into routines, diet, lifestyle, symptoms and quality of life. Validated questionnaires can support regulatory submissions, as well as bolster the impact of peer-reviewed publications.
A single timepoint sample only captures a snapshot of a patient, while a longitudinal study involving repeated sampling provides a more complete patient profile. Repeated measures of the same individual also bolsters statistical power and may require fewer enrolled patients. Overall, collecting diverse longitudinal data can provide valuable insights into disease progression, medication efficacy, and adverse events. Longitudinal sample collection may follow regular, predetermined intervals, or may be triggered by an event (such as symptom-based flare-ups or other disease characteristics). While the latter scenario can provide valuable insights in evaluating study objectives and developing diagnostics, these unscheduled study visits complicate study design and place additional burden and stress on patients, particularly when travel to study sites is necessary.
In-home collection with Sanguine empowers researchers to incorporate longitudinal design in their studies. By eliminating the travel requirement, participants are more inclined to consent to repeated measures, and they can easily arrange for event-triggered collections with their dedicated mobile phlebotomist. Additional unplanned collections from study participants due to unforeseen study changes or amendments can also be accommodated following reconsent of these patients. A recent trend in study data collection that complements in-home collection is the incorporation of biometric trackers or other wearable technologies to passively generate data that can help inform exploratory study endpoints.
Considerations for a precision medicine design approach
In recent years, the complexity of study designs has also been driven by the new era of therapeutic development focused on precision medicine. This approach harnesses genomic and molecular approaches to identify similar subpopulations within a heterogeneous disease to ultimately develop tailored treatments and interventions. Working with Sanguine can allow for faster recruitment of a greater number of participants in a particular subpopulation, given our mobile at-home approach that spans across the United States.
Precision medicine approaches are bolstered by the promise of big data collected by a variety of advanced technologies that can help inform efficacy endpoints. As such, collecting the right biological samples and other relevant data is extremely important. It is often presumed that more data will lead to better and more reliable results, but sometimes design limitations can actually magnify biases and lead to incorrect conclusions. New analytic approaches, relying on artificial intelligence and machine learning are further fueling the potential of personalized medicine development. This added complexity can make it even tougher to protect against erroneous conclusions, when the methods used to generate the results may be tough to scrutinize. As such, determining the ideal outcome measures for efficacy objectives and endpoints is required and for such studies, it may require highly specialized statisticians and researchers for the highest chances of success. Overall, current trends are showing a substantial push towards precision medicine approaches in study design. As exciting as this new era will be, it will also introduce much greater complexity into study design planning and execution to avoid poorly designed studies with inconclusive or erroneous results.
Other study considerations
Designing a clinical study must balance the desire for as much data as possible and the burden of participation on the patients. It can often be difficult to anticipate what questions you may want answered or what sample collection time points are optimal. Sometimes, evaluating whether the added complexity or sample collection time points are truly necessary can help improve patient participation experiences and increase recruitment and retention. At Sanguine, since all our sample collection methods are considered non-invasive and ensure patient comfort at home during each visit, we typically recommend collecting as many sample types at once that a researcher may anticipate needing for future analyses. We work with researchers to determine what specific samples are needed and we are also able to process many sample types, such as the isolation of PBMCs or further subtypes, in our partner laboratory facilities according to standard or even customized procedures. We are also able to perform a wide range of CLIA-certified diagnostic tests such as HLA typing, infectious disease panels or other genetic tests. Also, when designing more complex studies, regular communication between researchers and partner CROs is critical to maintain study consistency and integrity in execution. Taking a comprehensive approach that involves both internal and external subject matter experts to build and iteratively optimize the clinical study design will help to ensure successful execution of the clinical study. Lastly, with complex protocols, working with a central IRB can often help ensure rapid coordination of amendment reviews and adequate resources and expertise to address any safety or study concerns.
Conclusion
Proper study planning can dictate the difference between the success and failure of a long and expensive clinical study. Working with Sanguine gives you access to our expertise throughout the study start-up and execution phase of your research. We can provide comprehensive feasibility evaluations and provide our insights from previous experience to help proactively plan for and mitigate potential downstream scientific and operational risks associated with the study design. Making informed decisions during the planning phase of a clinical study can help to proactively reduce the risk of costly delays or improperly designed studies, which accelerates clinical development timelines to help bring potential treatments from the bench to bedside.
Overall, increased study complexity requires researchers to partner with organizations dedicated to maximizing study success and improving patient lives. At Sanguine, we understand the difficulties and challenges to study design, recruitment and execution and are committed to providing solutions to drive translational research forward. By leveraging our extensive patient community of >50,000 engaged, potential participants along with our streamlined mobile, at-home study visit model, we can provide our research partners with a customized approach to fulfill their study needs.
References
- Impact report: Faster New Drug Approval Times are More than Offset by Longer Clinical Times in U.S. July/August 2020. Tufts Center for the Study of Drug Development. Volume 22, Issue 4. Available at: https://f.hubspotusercontent10.net/hubfs/9468915/TuftsCSDD_June2021/images/Jul-Aug-2020.png (accessed June 20, 2022).