
Care About Rare
A PATIENT-LED APPROACH
TO RARE DISEASE RESEARCH

MISSION
To collect biospecimens from a diverse yet hard-to-reach patient population of adults and children with mucopolysaccharidosis (MPS) or allele carriers.

CHALLENGES
Meet patient enrollment goals targeted at a small, geographically dispersed rare disease patient population to satisfy aggressive sponsor-set study timelines.

SOLUTION
Take advantage of an upcoming two-day rare disease conference in San Diego, CA, to collect samples from consenting attendees.
Rare Disease Research Faces Unique Challenges
With approximately 30 million people living with roughly 7,000 identified rare diseases in the U.S., translating research to the clinic in this field faces more than a few unique challenges.¹ Although rare by definition, the disorders that make up this group are not rare to the patients and their families desperate for better treatment options.
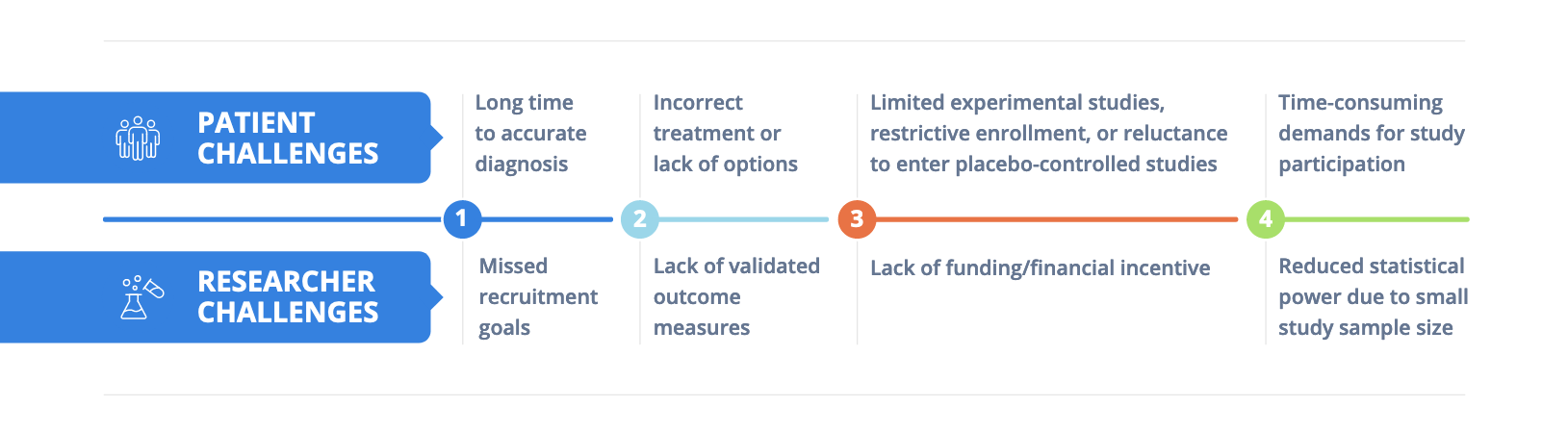
To date, about 5% of all rare diseases come with FDA-approved treatment options – even though the majority of such conditions substantially reduce life expectancy and quality of life – highlighting the serious unmet need for novel therapies.² In an analysis of 659 rare disease randomized control clinical trials conducted between 2010 to 2014, a total of 199 studies (30.2%) were discontinued, with lack of patient accrual representing the most common reason why (32.1%).³
On the upside, even with these challenges, advancements in molecular diagnostics and cutting-edge approaches to precision medicine are accelerating drug development. Though, executing clinical studies in this field requires creative, experienced, and multi-pronged strategies to drive their success.
Study Requirements
Participants
Commitment
Sample Processing
Up to 60 MPS patients and allele carriers who met study eligibility.
Patients were expected to be between 1 and 18 years of age (inclusive), while carriers were likely to be adults.
Collect whole blood, urine, and dried blood spot samples.
Follow-up dried blood spot samples were collected from a sub-cohort of the 60 enrolled patients.
Within 1 hour of collection, plasma and urine samples were processed, aliquoted, and frozen. All samples were shipped to a central lab facility by overnight courier.
Patients collecting dried blood spots at home were given instructions for shipment.
Rare Disease Research Requires A Rare Approach—
The Flexibility to Reach the Patient, Wherever They Are or May Go
Our innovative recruitment strategies are effective largely because of our ongoing relationships with the more than 100 patient advocacy groups and non-profit stakeholders in our national network. Through these partnerships, we work to gather insights that inform us on how best to serve patients' needs through biomedical research.
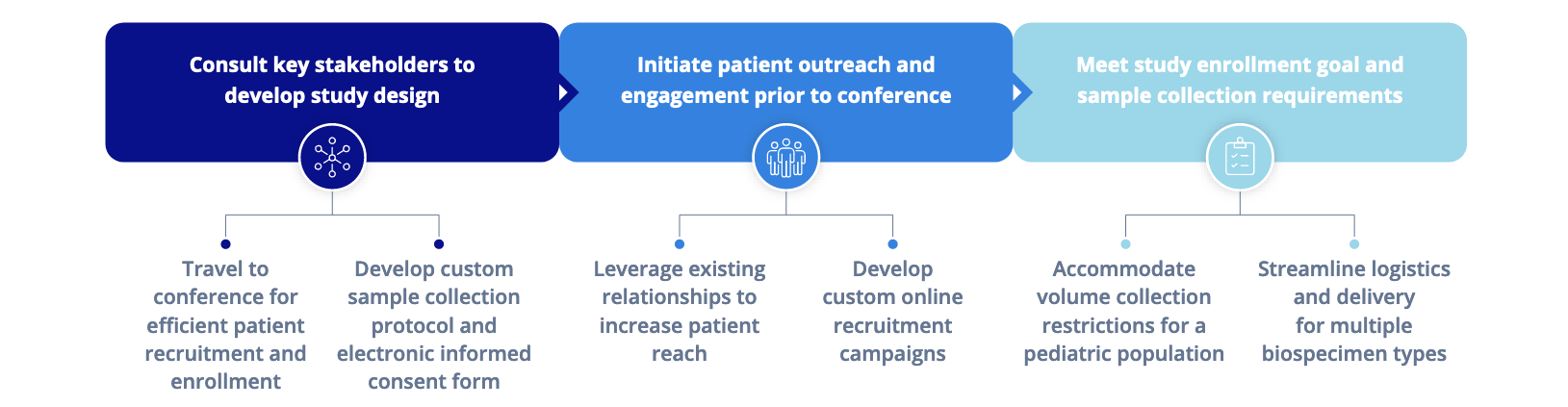
Rare disease research studies hinge on a patient-forward, decentralized approach to ensure the swift recruitment of eligible patients. We routinely adapt our model, addressing each research program's unique needs—from study design, recruitment, and execution to completion. In this way, we maximize success, reduce costs, and minimize failed or inconclusive rare disease studies.
REFERENCES
¹ Griggs RC, Batshaw M, Dunkle M, et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab 2009;96(1):20–26.
² Institute of Medicine (U.S.) Committee on Accelerating Rare Diseases Research and Orphan Product Development. Rare Diseases and Orphan Products: Accelerating Research and Development. Washington (D.C.): National Academies Press (U.S.); 2010.
³ Rees CA, Pica N, Moonuteaux MC, Bourgeois FT. Noncompletion and nonpublication of trials studying rare diseases: A cross-sectional analysis. PLoS Med 2019;16(11):e1002966