
Expanding the reach of clinical trials through benchmarking
Immunogenicity biomarkers in individuals previously infected with COVID-19 were used to
evaluate vaccines in preclinical and clinical trials.
Sahin U, et al. (2020) Nature. 586: 594–599.
Mulligan MJ, et al. (2020) Nature. 586: 589-593.
Walsh EE, et al. (2020) N Engl J Med. 383: 2439–2450.
Vogel AB, et al. (2021) Nature. 591: 283–289.
Sahin U, et al. (2021) Nature. 595: 572–577.

OBJECTIVE
Provide a convalescent sera cohort representing COVID-19-recovered individuals for evaluating biomarkers in vaccine trials.

STUDY
Sera from individuals aged 18-83 years who donated blood in their homes at least 14 days post-diagnosis and were asymptomatic at the time of collection.
INSIGHT
Immunogenicity of the lead vaccine candidates in preclinical and clinical studies could be compared directly against COVID-19-recovered individuals without needing clinical site biospecimen collection.
The rise of hybrid and decentralized clinical trials
The tools for achieving flexible trials (e.g., hybrid, decentralized, and direct-to-participant) have evolved into common practice, precipitated by disruptions to site-based specimen and data collection during the COVID-19 pandemic.1 By taking advantage of virtual tools and in-home visitation, trial sponsors can engage a voluminous and diverse patient population more efficiently, inclusively, and cost-effectively, regardless of participant geography or mobility.1,2 Sanguine’s nationwide network of patients and professional phlebotomists ensure that in-home, prospective studies are conducted reliably and compliantly according to predetermined protocols, especially when access to clinical sites is limited or burdensome.
Benchmarking biomarkers for vaccine and diagnostic development
A well-characterized human sample containing relevant biomarkers is often necessary to benchmark measurements and connect data streams from preclinical and myriad decentralized clinical sites.3 In evaluating multiple COVID-19 vaccine candidates, manufacturers coordinated with Sanguine to prospectively collect convalescent sera in-home from unimmunized symptomatic and asymptomatic patients. Vaccine-induced humoral and neutralizing antibody responses could be subsequently benchmarked against individuals who had recovered from COVID-19.
Study description
Ranging from 18-83 years of age, individuals in the Pfizer/BioNTech vaccine cohort donated blood in their homes at least 14 days post-diagnosis and were asymptomatic at the time of collection.4
Results and Conclusions
In preclinical results involving immunized rhesus macaques, the two lead mRNA formulations elicited tighter IgG binding to the Spike protein receptor binding domain (RBD) and neutralizing antibodies against SARS-CoV-2, compared to the unimmunized convalescent cohort.5
In subsequent clinical trials, the immune responses of immunized individuals were consistently compared to the at-home collected convalescent sera cohort (Figure 1). The two lead Pfizer/BioNTech vaccine candidates demonstrated robust and durable immunogenicity in the phase I/II trial after the second vaccine dose, compared to the COVID-19 recovered cohort.4,6,7
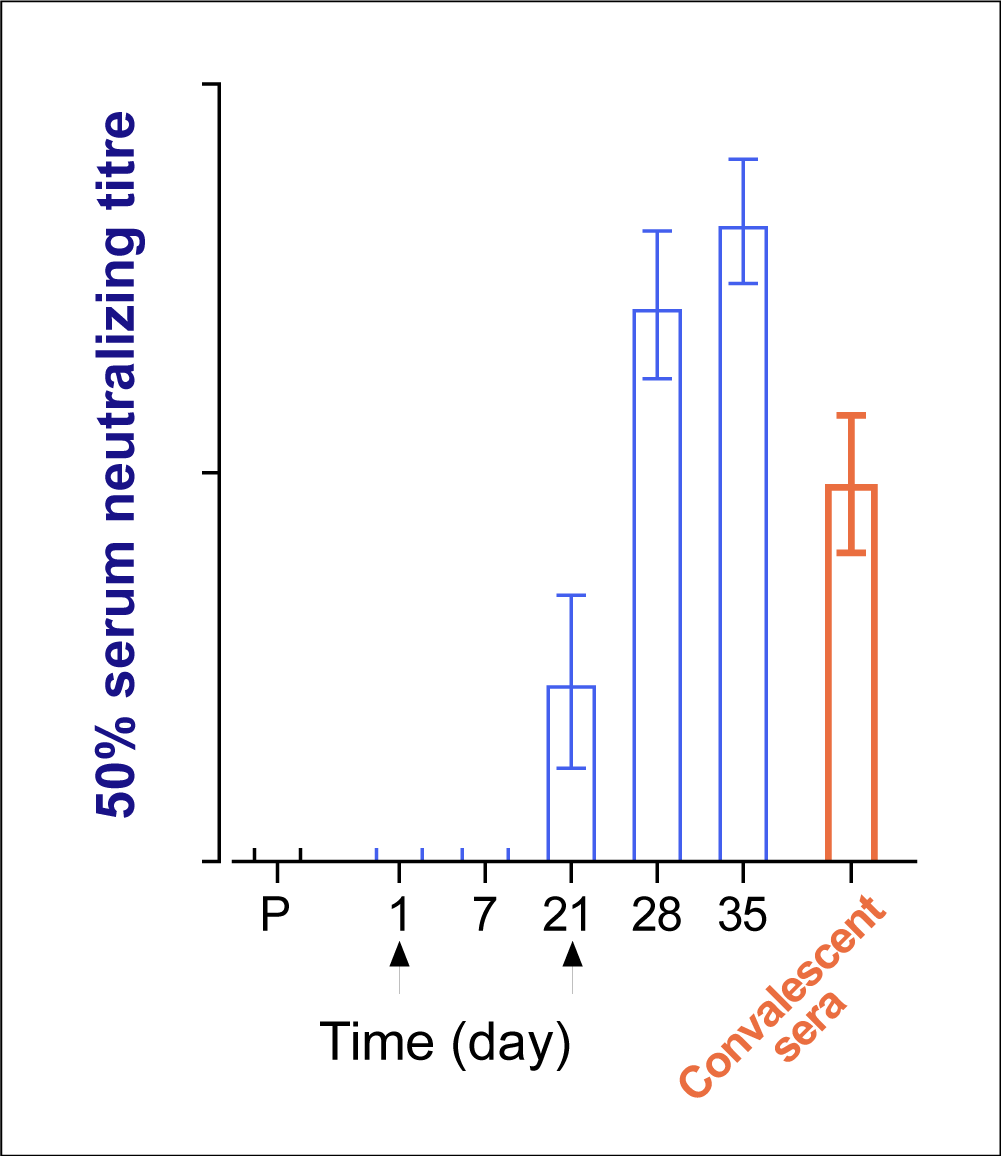
Figure 1. Representative plot of neutralizing antibodies
measured in immunized individuals compared to
recovered individuals in evaluating COVID-19 vaccines.
The two lead Pfizer/BioNTech mRNA candidates showed similar immunogenicity profiles, and BNT162b2 was chosen for the pivotal trial based on its favorable safety profile.8
Diagnostic companies took a similar approach to evaluate immunogenicity assays in yielding a unified COVID-19 diagnostic test.9 Thus, using a prospective, in-home collected population to represent a benchmark cohort is a powerful therapeutic and diagnostic development strategy.
References
1Anderson, M. How the COVID-19 pandemic is changing clinical trial conduct and driving innovation in bioanalysis. Bioanalysis 13, 1195–1203 (2021).
2van Norman, G. A. Decentralized Clinical Trials: The Future of Medical Product Development? Basic to Translational Science 6, 384–387 (2021).
3Folegatti, P. M. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet 396, 467–478 (2020).
4Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020).
5Vogel, A. B. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 592, 283–289 (2021).
6Sahin, U. et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595, 572–577 (2021).
7Mulligan, M. J. et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593 (2020).
8Walsh, E. E. et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. New England Journal of Medicine 383, 2439–2450 (2020).
9Bonhomme, M. E. et al. Robust validation and performance comparison of immunogenicity assays assessing IgG and neutralizing antibodies to SARS-CoV-2. PLoS One 17, e0262922 (2022).