
From Serum to Survey
FOLLOWING AN ACTIVE COVID-19
PATIENT MEMBER COMMUNITY OVER TIME
MISSION
To enhance and expedite patient recruitment for dozens of COVID-19 research studies.
CHALLENGES
To collect serum from recovered COVID-19 patients and track their vaccination status over time.
SOLUTION
Build a national mobile medical workforce of more than 100 phlebotomists to screen and enroll 1,000-plus patients with enhanced safety measures.
The Recruitment Challenge
Many study sponsors find themselves doubling study timelines simply to meet patient enrollment goals. But extending a study to focus on enrolling and retaining patients eats up a project’s time and funding. Worse, it delays the drug and vaccine development pipeline.
To that end, the coronavirus disease 2019 (COVID-19) pandemic has underscored the implications of urgency and speed for getting patients involved—research studies have held equal weight to the goal of developing validated diagnostic tests, effective vaccines, and other antiviral therapeutics. A massive shortage of accurate testing technologies and potent treatment options meant that there was no time to prolong meeting patient enrollment goals.
From the patient’s perspective, though, there are many hurdles to joining or staying in a research study. It may be hard to travel to the study site, or patients may be unable to use paid time off benefits to create participation time. They may have mobility or disability issues or be unable to deal with inconvenient study visit times. And compensation for participating may not adequately cover the financial losses a participant incurs if their time involvement conflicts with work.
A 2013 study by the Tufts Center for the Study of Drug Development1 found that on a global scale:
- 48% of study sites
miss their enrollment targets
- 11% of study sites
fail to enroll a single patient
- Most study sponsors
assume that 30% of
their participants will drop out
Bringing Research to The Patient
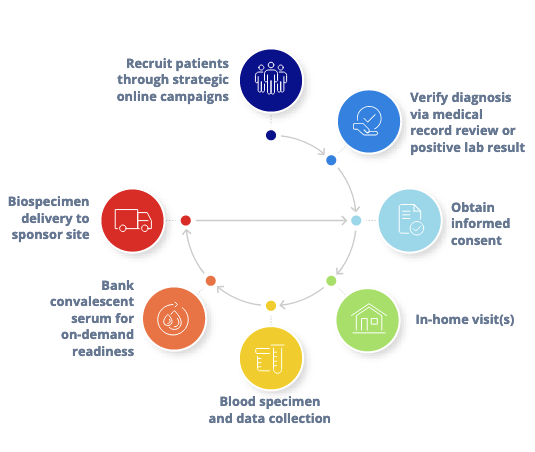
Our direct-to-patient research services model flips the script. Patients needn’t be burdened with reporting to study sites or medical centers when we have the power to bring a clinical or translational research study into a participant’s own home.

Our mobile medical personnel work with us remotely, in a flexible partnership that forms the backbone of our direct-to-patient services model. They strictly adhere to study protocols and use universal precautions and heightened infection control measures, including personal protective equipment, during all in-home visits.
Banking Convalescent Serum for COVID-19 Research
In response to the urgent need, we built and scaled a national workforce of 150 phlebotomists who served researchers by recruiting over 1,000 participants and collecting convalescent serum from more than 500 participants who answered our recruitment call.
INCLUSION CRITERIA
EXCLUSION CRITERIA
- Recovered from COVID-19 and between 18–100
years of age - Willing and able to provide written informed
consent and photo ID - Provide proof of COVID-19 positive PCR test result
- Pregnant or nursing females
- Excess blood loss, including blood donation
- Known history of HIV, hepatitis, or another serious infectious disease
- Previously taken an investigational product within the last 30 days
Through ongoing partnerships with leading pharmaceutical and biotechnology companies, such as Pfizer, EpiVax, Inc., and Vir Biotechnology, we’ve supported the completion of more than 25 COVID-19 research studies to date. Most recently, Pfizer researchers published the results of their phase 1 clinical trial in The New England Journal of Medicine, where convalescent serum samples collected from our nationwide reach of participants were used as a benchmark to make conclusions about vaccine immunogenicity.²
Tracking COVID-19 Through Observational Patient-Reported Data
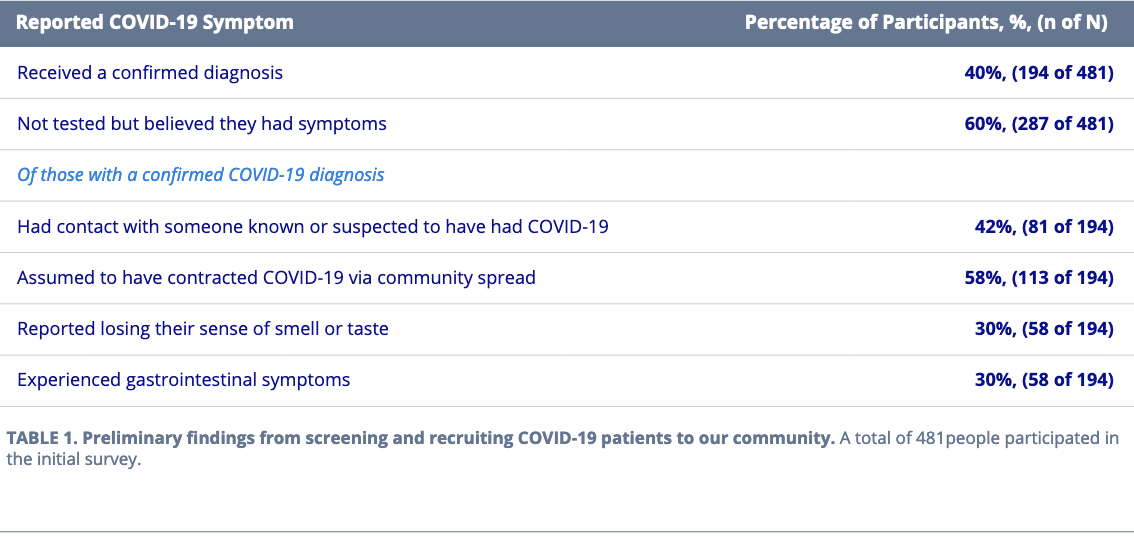
In the early months of recruitment, between March and May 2020, we noted some intriguing preliminary symptom and spread trends reported by our community of COVID-19 patient member(Table 1).
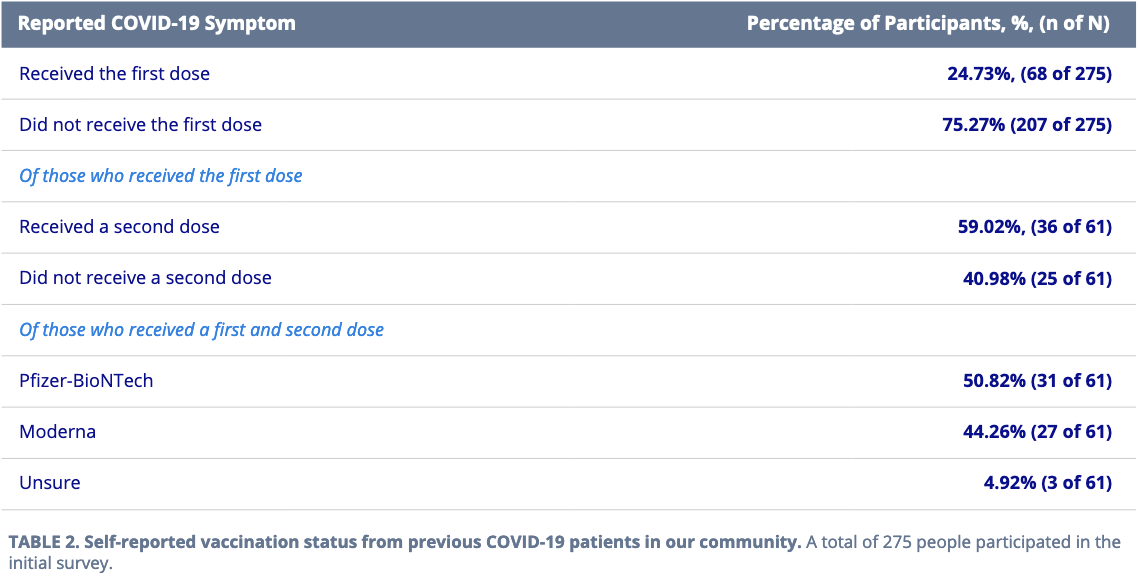
Given the industry’s vaccine development success throughout 2020, we’ve re-engaged our COVID-19 patient member community to track their vaccination status since contracting the disease (Table 2).
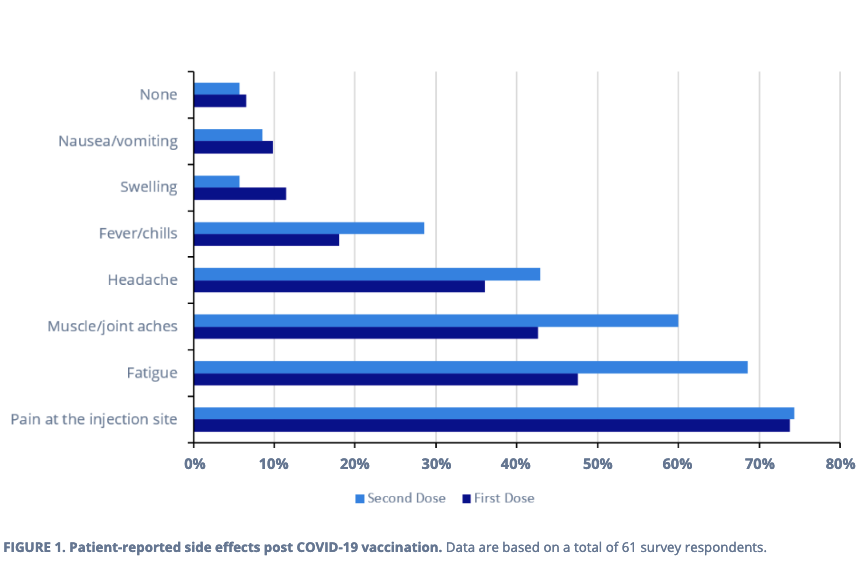
Moreover, those who have received the first and second vaccinations were also surveyed for side effects. Most respondents reported experiencing at least one side effect after receiving each dose, with a comparable proportion of people having pain at the injection site (~74%), nausea and vomiting (~9%), or no symptoms (~6%) with either dose (Figure 1). The number of respondents stating fatigue, fever and chills, and muscle and joint aches was approximately one-and-a-half times higher after the second dose when compared with the first. Finally, almost two times as many people noted swelling after the first vaccination compared with the second (Figure1).
As we continue to grapple with COVID-19 and the varying strains to come, exploring the health status from the patient’s perspective and integrating survey data in future study designs is required to ensure a patient-centered understanding of COVID-19 in the long-term. Our community-building approach is patient-centered and allows us to engage with COVID-19 patients over time to create datasets that are relevant to researchers, patients, physcians, and policymakers alike.
REFERENCES
¹ Kaitin KI, editor. 89% of trials meet enrollment, but timelines slip, half of sites under-enroll. Tufts Center for the Study of Drug Development Impact Report. 2013;15(1).
² Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906


