
Describing the patient experience in biomarkers
A prospective, longitudinal study using in-home collected biospecimens identified a panel
of predictive biomarkers.
White et al. (2022) Br J Haematol. 196(4):1052–1058.
Tarasev et al.(2022) medRxiv. https://doi.org/10.1101/2022.10.20.22281335
Hines et al. (2021) Br J Haematol. 194: 1074–1082.
Pittman et al. (2021) Blood. 137(15): 2010–20.

OBJECTIVE
Identify functional and clinically relevant biomarkers predictive of symptom onset and progression.

STUDY
Blood samples were collected in-home from 35 study subjects with Sickle Cell Disease (SCD) every three weeks for baseline and during self-reported vaso-occlusive crises (VOCs). Daily electronic patient-reported outcomes (ePRO) and continuous activity via a wearable device were also obtained.
INSIGHT
A flow-based adhesion biomarker panel predicted VOC onset and duration while correlating strongly with SCD severity and treatment status. The assay may be clinically valuable in stratifying patients for evaluating SCD therapies.
Biomarker identification outside the clinic
Identifying biomarkers that chronicle the natural history of a disease and treatment response constitutes a primary goal of translational precision medicine. Yet, much of the patient experience occurs outside the clinical setting. Flexible trial design (e.g., decentralized, direct-to-participant, and hybrid clinical trials) features the dual patient-centric goals of reducing the burden of participation while increasing the collection of real-world evidence and biomarkers.1 Prospective biospecimen collection enables the at-home recording of analytical and digital biomarkers from predefined patient populations longitudinally, extending the patient experience beyond the clinic.
Sickle Cell Disease and Vaso-Occlusive Crises
Sickle Cell Disease (SCD) is commonly associated with unpredictable and debilitating severe pain episodes called vaso-occlusive crises (VOCs). While VOC frequency and severity correlate with SCD morbidity and mortality, the VOC clinical endpoint relies exclusively on the patient visiting a medical facility for treatment, grossly underreporting the patient experience. To capture the natural history of SCD-associated VOCs, investigators partnered with Sanguine to implement the Evaluation of Longitudinal Pain Study in Sickle Cell Disease (ELIPSIS).
This non-invasive, prospective study cataloged the patient experience through continuously measured actigraphy, daily electronic patient-reported outcomes (ePROs), and longitudinal exploratory and established clinical assays to identify meaningful blood and digital biomarkers for future treatment investigations.2
Study description
Blood samples were collected in-home from 35 study subjects with Sickle Cell Disease (SCD) every three weeks for baseline and during self-reported vaso-occlusive crises (VOCs). Daily electronic patient-reported outcomes (ePRO) and continuous activity via a wearable device were also obtained (Figure 1).2
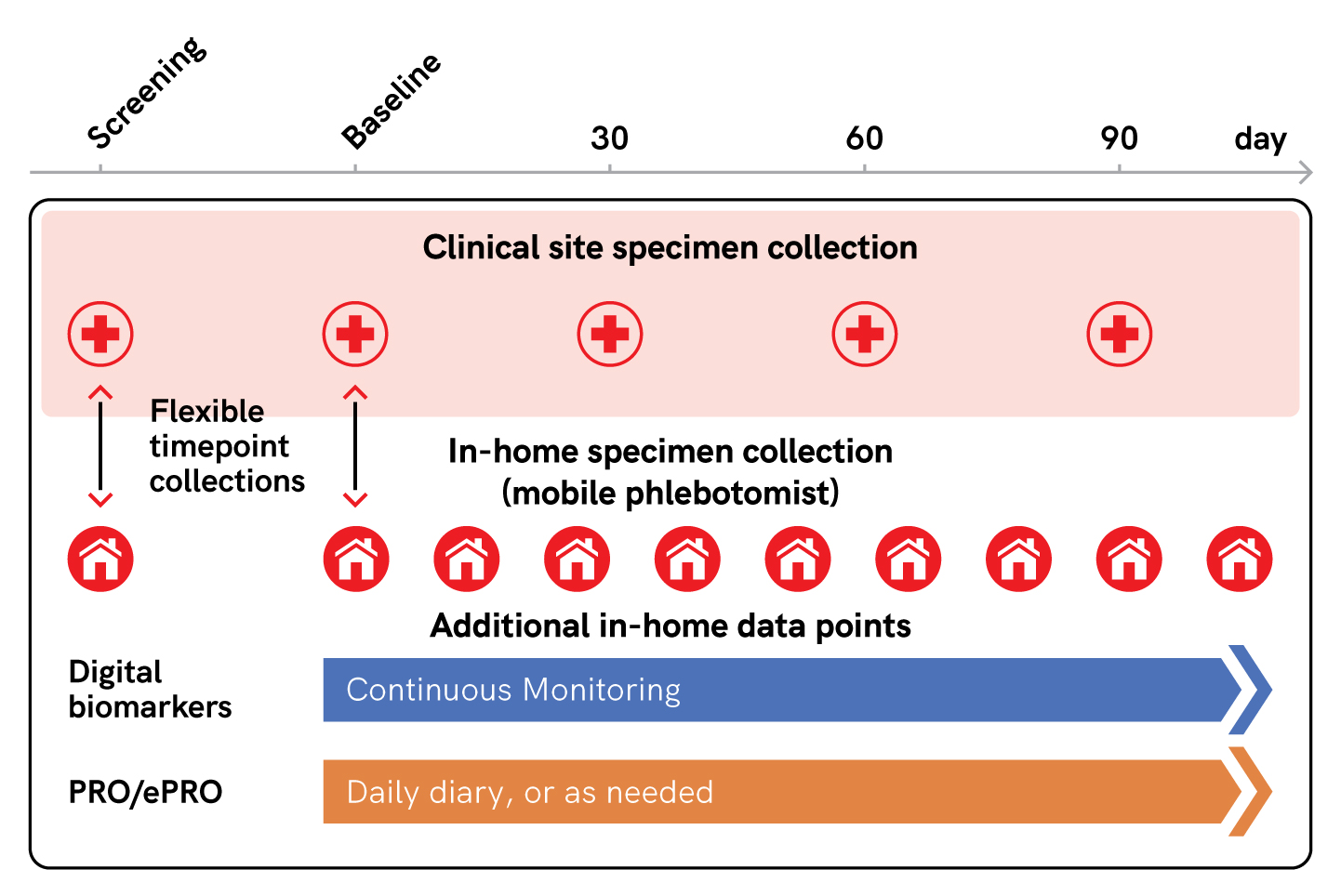
Figure 1. Concept of a flexible trial, inspired by the ELIPSIS Study.¹
Results and Conclusions
ELIPSIS demonstrated the feasibility of prospectively collecting diverse and longitudinal biomarker streams associated with VOCs that may represent new endpoints in SCD clinical trials.2
Longitudinal blood collections associated with VOCs reported outside a medical facility enabled the identification of flow-based adhesion assay biomarkers predictive of a patient’s risk for experiencing a VOC.3
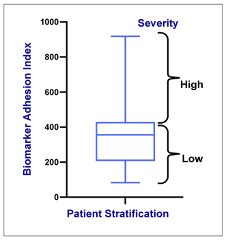
Figure 2. Stratifying SCD patients by VOC severity, which varies directly with the biomarker panel.
The resulting standardized clinical test correlated strongly with SCD severity, VOC onset, and treatment status, indicating assay utility for patient stratification in clinically evaluating SCD therapies.4,5
Reports from ELIPSIS suggest that the patient experience can be captured through biomarkers outside a clinical setting and could be broadly applied to other disease conditions.
References
1van Norman, G. A. Decentralized Clinical Trials: The Future of Medical Product Development? Basic to Translational Science 6, 384–387 (2021).
2Pittman, D. D. et al. Evaluation of Longitudinal Pain Study in Sickle Cell Disease (ELIPSIS) by patient-reported outcomes, actigraphy, and biomarkers. Blood 137, 2010–2020 (2021).
3Hines, P. C. et al. Flow adhesion of whole blood to P-selectin: a prognostic biomarker for vaso-occlusive crisis in sickle cell disease. Br J Haematol 194, 1074–1082 (2021).
4White, J. et al. Longitudinal assessment of adhesion to vascular cell adhesion molecule-1 at steady state and during vaso-occlusive crises in sickle cell disease. Br J Haematol 196, 1052–1058 (2022).
5Tarasev, M. et al. Adhesion to VCAM1 and P-selectin Predict Time-to-Resolution (TTR) of Vaso-Occlusive Crisis. medRxiv. 2022.10.20.22281335. (2022)