
Process Development: Replicating The Final Cell Therapy Product
Disease-state leukopaks are effective surrogates for establishing cell therapy manufacturing parameters.
![]()
CHALLENGE
Leukopaks from patients with confirmed disease are preferred for establishing process development parameters; however, identifying such candidates for apheresis is arduous.
![]()
SOLUTION
Leukopak donors with confirmed diagnoses (n=5 x 2 conditions) were recruited from Sanguine’s 70,000+ patient community to support CAR-T development programs in two distinct autoimmune disease indications.
OUTCOME
Access to leukopaks more closely resembling the final autologous therapy product given to patients enabled the process development team to obtain more accurate data in promoting two autoimmune disease programs.
Challenges abound in autologous cell therapy manufacturing
Adoptive cell therapies like CAR-T have proven effective, potentially curative treatments for certain blood cancers, and the technology is being applied to autoimmune diseases, including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), multiple sclerosis (MS), and systemic lupus erythematosus (SLE). Given these modalities involve extracting a patient’s own cells, engineering and expanding them outside the body into a safe and reproducible “living drug” to be readministered to the patient, an entirely new pharmaceutical science referred to as process development has evolved to manage, optimize, and ensure the quality of the manufacturing process.
Process development scientists labor to emulate the clinical manufacturing of the cell therapy product at every stage of the drug pipeline. The immune cells engineered into disease-fighting drugs often are in low circulating abundance. For example, regulatory T cells (Tregs; e.g., CD4+CD25+FoxP3+ cells), a popular target for autoimmune cell therapy, comprise only ~1% of peripheral T cells. Isolating Tregs by apheresis is needed as a first step before fluorescence-assisted cell sorting.¹ Therefore, process development teams often use lymphocyte-enriched apheresis products from human donors (a.k.a. “leukopaks”) as surrogates for establishing process development parameters in anticipation of the “real thing” coming from patients in clinical trials.
Most leukopaks come from healthy donors; however, patients with autoimmune conditions typically present with different immune cell profiles than healthy-matched controls (Figure 1). Peripheral blood immunophenotype distributions can distinguish Crohn’s disease and ulcerative colitis patients², RA and Spondyloarthritis at early stages of disease³, and correlate with disease progression and treatment response in MS4. Thus, access to leukopaks from patients with confirmed disease can provide a more accurate representation of the lymphocyte apheresis material from patients receiving the autologous treatment.
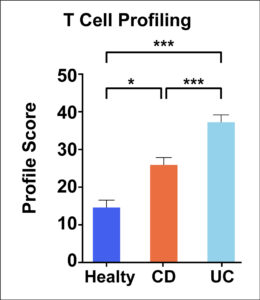
Figure 1. Representative data demonstrating how T cell profiles from healthy controls, patients with CD, and patients with UC all significantly differ with respect to each other. Adapted from Bai and colleagues.5
Supporting Process Development with Disease-State Leukopaks
Client:
Process development team lead at a biotechnology company developing autologous CAR-T therapies to treat various autoimmune diseases.
Request:
10 total leukopak donations, two autoimmune conditions of 5 patients with confirmed diagnosis.
Outcome:
Before working with Sanguine, the process development team relied on leukopaks from healthy donors to establish manufacturing parameters such as genome engineering, cell expansion, and cryopreservation. Access to leukopaks from patients with the same autoimmune conditions the therapy programs aim to treat enables the process development team to achieve more accurate analytical data related to manufacturing quality.
Please contact us to find out whether Sanguine can support your cell & gene therapy development program.
References
1Janssens, I. & Cools, N. Regulating the regulators: Is introduction of an antigen-specific approach in regulatory T cells the next step to treat autoimmunity? Cellular Immunology. 358, 104236 (2020).
2Mitsialis, V. et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology. 159(2), 591-608.e10 (2020).
3Wang, J., Xue, Y. & Zhou, L. Comparison of immune cells and diagnostic markers between spondyloarthritis and rheumatoid arthritis by bioinformatics analysis. J Transl Med. 20, 196 (2022).
4Tenient-Serra, A., Ramo-Tello, C., & Marinez-Caceres E. M. Immunomonitoring Lymphocyte Subpopulations in Multiple Sclerosis Patients. In: Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Ian S. Zagon and Patricia J. McLaughlin (Editors), Codon Publications, Brisbane, Australia (2017).
5Bai X., et al. Immune Cell Landscaping Reveals Distinct Immune Signatures of Inflammatory Bowel Disease. Front Immunol. 13, 861790 (2022).