Leukopaks and LeukoLots™: Healthy and Disease State
High-quality, RUO apheresis products
from our diverse network of research-ready donors
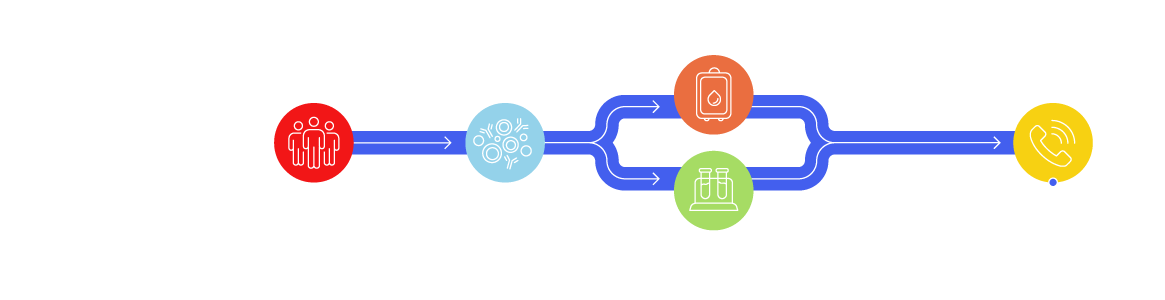
Leukopaks: One Donor, Billions of White Blood Cells
Large volumes of white blood cells can be separated by apheresis (leukapheresis) while plasma and red blood cells are returned to the donor. Research Use Only (RUO) leukapheresis products collected from the same donor provide a reliable and consistent source of up to 10 billion immune cells, including peripheral blood mononuclear cells (PBMCs), rare CD34+ cells, for biomarker, immunology, autologous, and allogeneic cell therapy research and product development.
LeukoLots™ are isolated PBMCs from a leukopak that are aliquoted and cryopreserved. Screening vials are available to ensure you choose the right LeukoLot for your research needs.
Process Development

Cell & Gene Therapy
Assay Validation
Rare Cell Isolation
Leukopaks from our network of 70,000+ donors

Diverse Donor Network
- 70,000 patient and healthy donors nationwide
- Confirmed disease diagnosis via medical record
- Medical histories
- Inclusion/Exclusion criteria
- Mechanisms to screen donors for distinct biomarker signatures

Comprehensive Donor Data
- Annotated medical records
- Study-specific questionnaires
- Infectious disease status
- HLA typing class 1 & 2
- Complete blood count (CBC)
- Total Nucleated Cell Count (TNC)
- Demographics

Quality
- Adherence to standards and protocols ensures high cell viability & recovery
- Certificate of Analysis (view a sample copy)
- Same-day or next-day shipment
- Up to 10 billion (full) or 5 billion (half) total nucleated cells per leukopak

Recallable Donors
- Longitudinal studies with multiple time points
- Concurrent collection of additional sample types (e.g., urine, stool, skin tapes)
- Nationwide network of qualified apheresis clinics
Additional characterization & isolation capabilities, including:
Mononuclear cell isolation (PBMCs, T, B, and/or NK cells)

100+ additional CLIA-certified diagnostic tests
Percent abundance characterization of immune cell subtypes
Trust Sanguine for Your Human Sample Research Needs:
All Sanguine locations maintain the appropriate certifications and registrations that demonstrate our commitment to excellence and continuous quality improvement.


More than Biospecimen Procurement
Take advantage of our experienced and dedicated sample collection, logistics, processing, characterization, and storage capabilities to optimize and streamline your study design and mitigate unnecessary variability.
Publications using Sanguine PBMCs:
Request A Quote for Sanguine Leukopaks
To provide you with an accurate quote, please complete the short form.
Alternatively, to speak with someone directly, please contact:
Phone: 818-462-8290
Email: LearnMore@sanguinebio.com