Building Confidence for First-in-Human Trials
Blume E, et al. (2021) Clinical & Translational Immunology
OBJECTIVE
Establish pharmacodynamics of a promising lead therapeutic candidate in donor whole blood from patients with target indication prior to first-in-human phase I study.
STUDY
Whole blood and medical records from adults with multiple sclerosis (MS) without history of taking B-cell depleting therapy and healthy matched controls.
INSIGHT
Target engagement and mechanism of action measurements were similar in whole blood from patients with MS compared to preclinical mouse and healthy human results, providing
confidence to continue with a phase I trial.
Preclinical human samples inform on drug pharmacology
Translation of biological discovery into therapeutics often involves identifying a protein target and screening it against chemical libraries, followed by preclinical pharmacology in human cells and animal models. To increase confidence prior to first-in-human studies, drug developers increasingly use human donor biospecimens from diseased patients (e.g., whole blood, PBMCs, and tissue biopsies) to test lead compounds in pharmacokinetic and pharmacodynamic (PK/PD) assays.
Treating multiple sclerosis by blocking pro-inflammatory B-cell activities
Bruton’s tyrosine kinase (BTK) is a key regulator of B-cell and myeloid cell activation and effector functions, acting as the downstream signaling node of both the B-cell receptor (BCR) and the Fc region receptor (FcR), respectively. BTK represents an attractive target for MS, given that BTK inhibition is neither cytotoxic nor depletive of B-cells.
Bame and coauthors tested a highly selective and reversable BTK inhibitor (BIIB091) in mouse and human preclinical models to determine pharmacokinetic and pharmacodynamic properties prior to phase I trials.
Sanguine Study Description
Whole blood and medical records for 6 adult subjects with multiple sclerosis, as well as 3 matched control subjects, were collected in-home and shipped within 48-hours to the client laboratory. For I/E criteria, patients were excluded if they had been taking certain MS or B-cell depleting therapies, as well as history of infectious disease.
Results and Conclusions
In preclinical mouse models and healthy human blood and PBMC assays, BIIB091 showed robust target engagement (BTK autophosphorylation) and selective mechanisms of action (blocking B-cell activation and myeloid cell function via BCR- and FcR-dependent mechanisms).
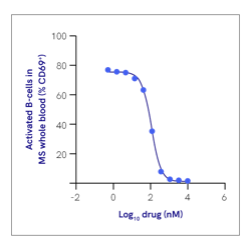
In whole blood from MS patients and healthy-matched controls procured from Sanguine, BIIB091 maintained high selectivity for inhibiting B-cell activation in patients with MS (Figure 1), a critically important milestone for clearing the compound for a first-in-human clinical trial.
BIIB091 safety, tolerability, pharmacokinetics, and pharmacodynamics were evaluated in a randomized, blinded, placebo-controlled, dose-escalation phase I clinical trial in healthy adults. Data indicated the compound blocked B-cell inactivation with similar pharmacology observed in the preclinical results.
Figure 1. Lead compound inhibition of B cell activation in whole blood from patients with MS.
Bame E, et al. (2021). Next-generation Bruton’s tyrosine kinase inhibitor BIIB091 selectively and potently inhibits B cell and Fc receptor signaling and downstream functions in B cells and myeloid cells. Clinical & Translational Immunology. e1295. doi: 10.1002/cti2.1295