
Hybrid by Design: Patient
Centricity in Clinical Trials
DUCHENNE MUSCULAR DYSTROPHY
MISSION
To conduct remote visits for a 4.5 year-long Phase 1 safety study in collaboration with a biopharmaceutical sponsor, two research hospital study sites, and a large contract research organization.
CHALLENGES
- Ease participation burden for DMD patients and families enrolled by carrying out more than 17 in-home sample collections over 4.5 years.
- Accommodate regular site-requested ad hoc in-home visits with less than a 5-day lead time.
SOLUTION
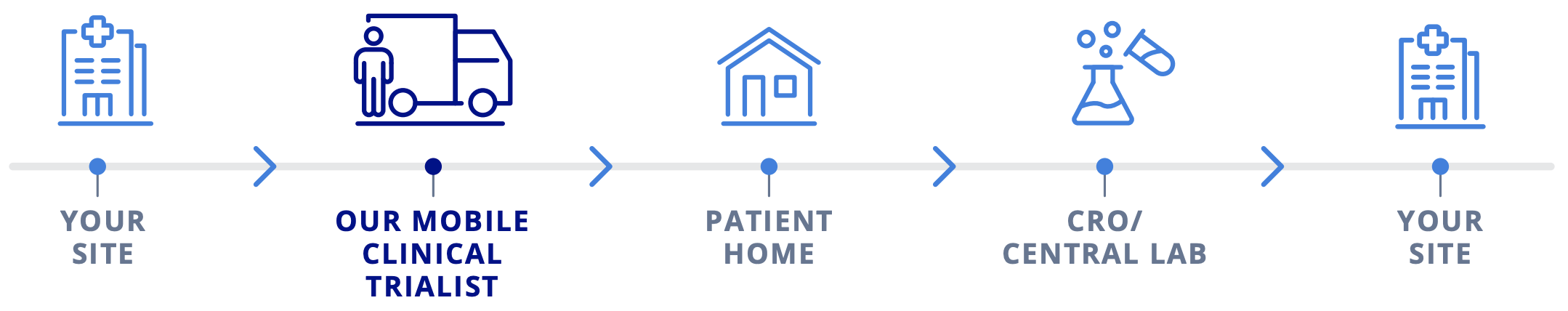
Collaborate on a hybrid clinical trial design, functioning as the sponsor’s study site extension. We coordinated 12 of the 17 scheduled visits to be remote and in-home. Sample collections were carried out as per study protocol and sent to a large contract research organization central laboratory for clinical testing.
Advances in Gene Therapy Bring Hope
As an X-linked disorder, Duchenne’s muscular dystrophy (DMD) primarily affects males, with an incidence of one in every 3500 males.¹ Today, more than 40 companies are running clinical studies aimed at bringing novel therapeutics to market for DMD—a significant improvement from roughly 10 years ago where only three companies had ongoing therapeutic studies.² The therapeutic landscape looks promising for many mutations associated with DMD, with three FDA–approved gene therapy drugs that partially repair some mutations in the faulty dystrophin protein, decreasing disease severity and slowing progression.
Decentralizing Trials to Be More Patient-Focused

DMD is the most common fatal pediatric disorder, with a life expectancy of approximately 20 years and a rapid deterioration in mobility and health during the early years of life.³ To that end, many parents are eager to participate in therapeutic studies. Yet, the physical, emotional, and financial challenges associated with DMD combined with clinical trial travel demands deter their participation. Indeed, a particular challenge for clinical researchers is successfully enrolling pediatric populations and maintaining long-term study commitments.
Rare disease patients receive specialized services and access to clinical trial opportunities through various institutional epicenters of care. As such, multi-centered site recruitment approaches are increasingly common to drive higher patient enrollment. However, study sites are often located at far distances from patients’ homes. Many patients live in more rural areas with limited accessibility, pointing to the need for a hybrid approach that combines in-clinic and in-home visits
Study Requirements
Participants
~24 DMD patients
Commitment
4.5-year study of an investigational therapeutic
Downstream Analyses
Blood and/or urine; varied between scheduled and unscheduled visits
Our Solution: Streamlining Clinical Trials by Site Extension
Meeting the demands of rare disease clinical trials often requires a bespoke study design that focuses on easing the burden of participation for patients and their families. We function as a strategic partner through remote site extension with capabilities that add valuable insights to clinical trial designs while delivering unique patient and family perspectives for optimized study performance.
Our patient-forward approach is built on direct relationships with patients and advocacy groups that empower participation in research. As a diverse and experienced team, we collect various biospecimen types and patient-reported data and work to exceed industry standards.


