Researchers
Community Access
Partner with Patients
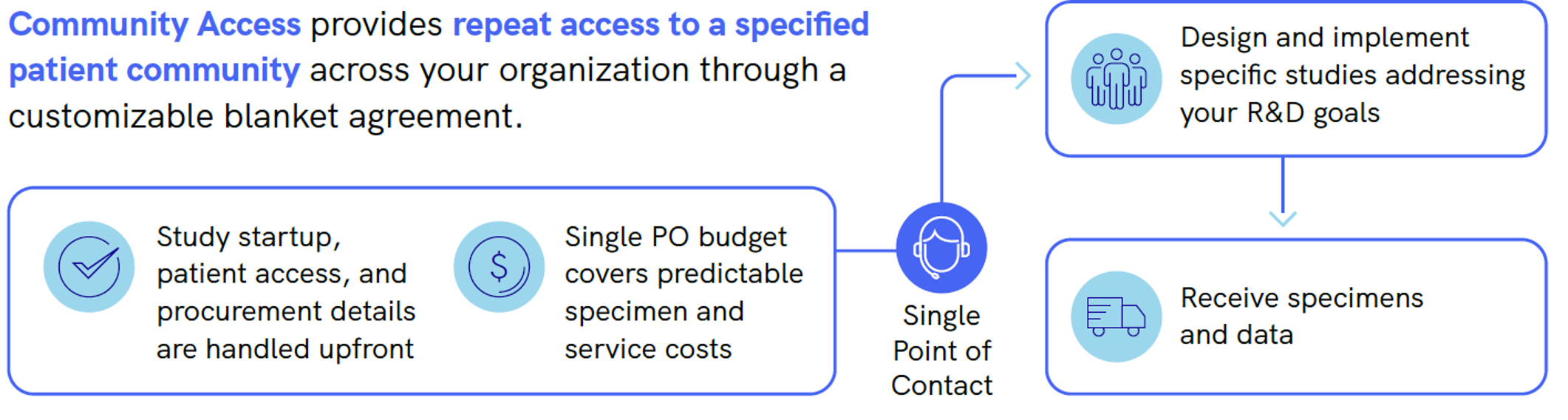
What is Community Access?
Streamline Study Fulfillment
Does your organization annually engage in multiple translational studies within one or several specific disease states?
Community Access may be right for you.
For researchers: Tackle multiple study designs in one or more specific disease states. Study designs can be dynamic, longitudinal, or cover different I/E criteria. Your community access grows as our Patient Network grows.
For procurement officers: Take care of all the administrative and financial requirements ahead of time to save your research team’s time and budgets. Eliminate redundancy in overlapping needs of research stakeholders.

QUALITY
Standard protocols that include 24 hour processing turnaround time

CONVENIENCE
Save time and increase quality with one vendor

EXPERIENCE
10+ years of laboratory processing experience
Take care of all your organization’s healthy and disease-state biospecimen needs
Combining Community Access covering one or several disease states with a healthy Onsite Employer Collection program enables your research teams to procure the most relevant and highest quality biospecimens when and how you need them.
These two programs offer convenience and customization in obtaining disease state and healthy specimens to cover any number of translational study designs.
Compliance & Quality Assured
Sanguine utilizes two internationally-recognized IRBs (Advarra and WCG IRB) for review and approval of all study protocols, specific study documentation, and study participant facing information. Both Advarra and WCG IRB are organized and operates in compliance with: FDA, OHRP, and International Conference on Harmonization (ICH).
Sanguine operates under an electronic informed consent process which executes 21 CFR part 11 compliant e-signatures, and Sanguine systems interacting with study participant information are HIPAA-compliant.
Everything you need for biomarker development and validation
Explore our service offerings to cut timelines and costs, reduce variability, and translate your data into insights.